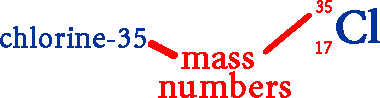|
The mass of an atom is almost entirely due to the mass of its neutrons and protons. The mass of an electron (9.11 x 10–31 kg) is 1/1800th of the mass of both a proton (1.673 x 10-27 kg) and a neutron (1.675 x 10-27 kg). As a result, the mass of the electrons factors little in the the total mass of the atom (it would take over 1800 electrons to equal the mass of 1 neutron). The mass of the protons + the mass of the neutrons is an element's mass number. Since isotopes of an element have different numbers of neutrons, they have different mass numbers. For example: Two naturally occurring isotopes of chlorine are chlorine-35 & chlorine-37. Thirty-five and thirty-seven are the mass numbers for the two isotopes. Both isotopes have the same number of protons (17).
The number of neutrons is determined by subtracting the number of protons from the mass number; (35-17 = 18 & 35-17 = 20 respectively).
Isotopes are also written using their symbol with the mass number (to the upper left) and atomic number (to the lower left).
|